The work just started…!
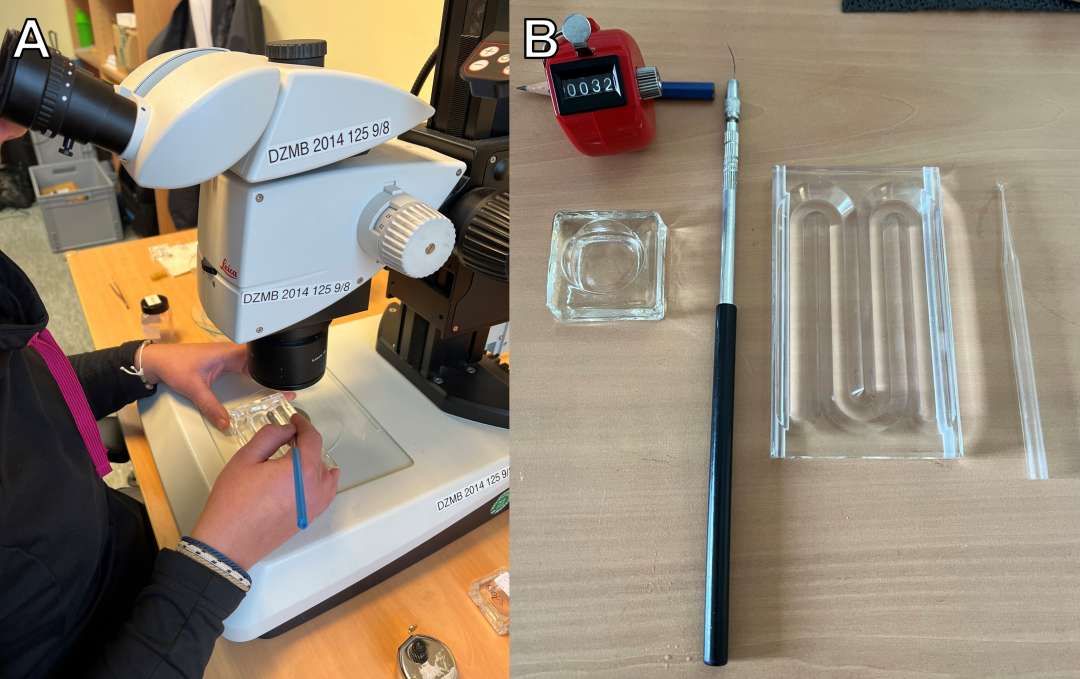
Working with the Multicorer and taking the samples during the expedition was just the beginning of the investigation. With these samples we hope to shed some light on the distribution and diversity of meiofaunal organisms, in my case on harpacticoid Copepoda. While we (Team DZMB) arrived in the middle of September, our sediment samples needed some more time. The first batch of our samples arrived at the end of October, and we started processing on the 1st of November. With processing, I mean following certain steps, like sieving, washing and balancing the weight for centrifuging. And by us, I mean my dear college Franzi Iwan and myself (Fig. 1). We must centrifuge our samples to separate organic material from the sediment. This step allows us to retain more organic material and organisms, as with other known methods. While we started with the samples preserved in ethanol, we did not have to stain our samples with rose Bengal, as we usually do it with samples preserved in formalin. This staining helps us to detect the organisms retained from the sediment. After centrifuging was done, we immediately started to sort samples. Again, a time-consuming task. Equipped with fine needles, stereo microscopes and special sorting trays for meiofauna, we extracted all target organisms (e.g., Copepoda, Kinorhyncha, Tardigrada) – slowly but steady (Fig. 2). All target organisms were transferred into block bowls, filled with ethanol and some drops of glycerol. The ethanol slowly evaporates, leaving the organisms unharmed in glycerol. Some might think we are done preparing the organisms for identification – but you guessed right – we are not. Before identification, it is crucial to mount the organisms on microscope slides. You take on slide, stick one or two – depending on the “size” of the Copepoda – reinforcement rings onto it and put a small drop of glycerol in the middle, then you can start to mount the organism. The Copepoda should lay on its back, presenting its legs and then – if it’s meant to be – you can place a cover slip onto it while the Copepoda still lays on its back (Fig. 3). A troublesome and time-consuming task, which sometimes must be repeated. As for yesterday, we finished with mounting all the Copepoda, so this task is done and the most important one can start – identification.
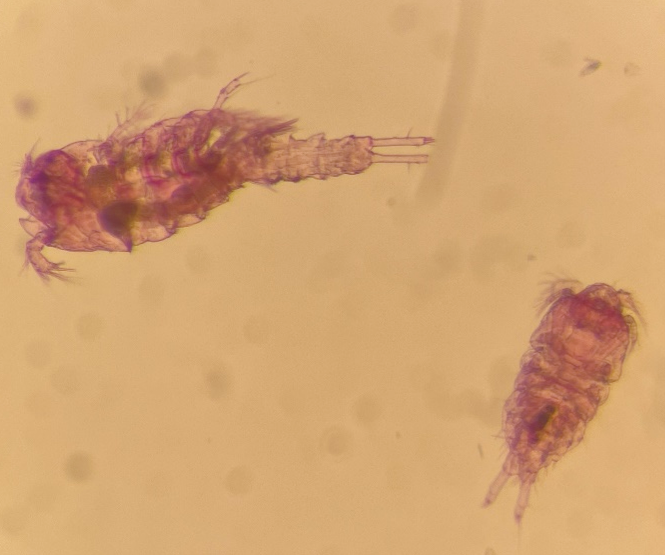
Now we are sitting on approx. 390 microscope slides, some with juvenile Copepoda, aka Copepodide, and some with full “grown” adult Copepoda (Fix. 4). This task is the most difficult and time-consuming, but also the most interesting one.